Masterclass Webinar Series
The challenges of IHC standardization
Started October 26, 2023
#VisiopharmIHCMasterclass
An impending paradigm shift in immunohistochemistry (IHC) quality assurance (QA) is becoming evident, driven by new data showcasing, and for the first time also measuring, ongoing significant inter-laboratory discrepancies in IHC. These discrepancies starkly exceed those noted in serum or plasma immunoassays. A recent editorial in the Archives of Pathology & Laboratory Medicine advocates for a revision of IHC QA regulations, suggesting a transition of regulatory practices from clinical immunoassay frameworks to IHC. Such arguments gain further traction with recent amendments to the ISO15189 standards.
In response to this, we are excited to announce the launch of a live webinar series of Masterclasses. The focus of these classes will revolve around the themes of standardization and quality assurance in IHC. The sessions are designed to share new information and help educate scientists, lab managers, and pathologists in the IHC lab about the recent findings and developments within the IHC community.
We invite you join us for this special series, where a stellar lineup of speakers will present their knowledge and experiences in ensuring high-quality IHC stains.
Topics to be covered in the series
The complete agenda will be released soon.
Regulatory shifts in IHC quality assurance
Recently, an editorial in Archives of Pathology & Laboratory Medicine, titled “Immunohistochemistry Should Be Regulated as an Assay”, and authored by Barbarajean Magnani and Clive Taylor, called for a transformative approach to immunohistochemical (IHC) analyses. The goal is to regulate IHC as an assay rather than a stain to improve diagnostic decisions. Learn from Clive Taylor about the reasoning behind the call for action and discuss its implications.
Clinical implications of IHC standardization deficiencies
External Quality Assessment (EQA) entities are crucial pillars for maintaining the consistency and precision of IHC staining protocols. As we edge closer to new regulatory adaptations, the role of EQAs will intensify. Presently, a pervasive absence of standardization manifests as variability in IHC staining, risking incorrect diagnoses and jeopardizing patient care. Delve deeper into this issue through insights from leading EQA's.
Innovative approaches to IHC staining quality - Ready for today
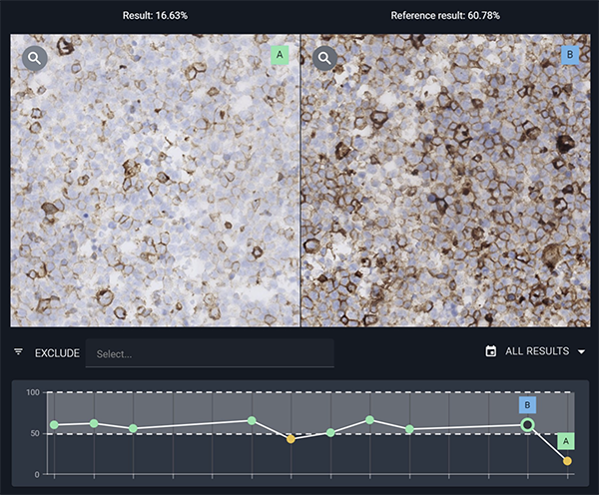
Quality of staining is integral to precision pathology, and support both manual and AI-driven interpretation. Discover how our AI-driven solutions are facilitating statistical process control for IHC. By adopting these new solutions, pathology labs can not only align with imminent regulatory frameworks but also gain actionable insights into their entire system, rectifying discrepancies ranging from equipment issues to protocol inconsistencies.
Streamlining deployment within laboratory workflows
Join pioneers and thought leaders as they discuss their experiences on integrating Qualitopix into their laboratory workflows and share their first data. Learn about their findings and how adding daily quality assurance drastically uplifts their staining consistency, support ISO accreditation, and optimizes lab resources.
Partnerships and collaborators
AI's potential in consistently monitoring and documenting staining consistency rests on the utilization of standardized reference materials. The last decade has witnessed substantial progress in this domain. Engage with various industry leaders to explore and understand new reference standards, and their critical role in process control within IHC workflows.
The road ahead for precision pathology
Automating analysis while generating reports on both patient-centric evaluations and IHC controls signals a significant leap for Precision Pathology. This evolution aligns with regulatory directives and facilitates the decentralized, scalable deployment of predictive assays worldwide, aiming to mitigate risks that might compromise patient care.
Engage with a global IHC community
Foster connections with a global group of IHC experts, researchers, and professionals. Engage in stimulating discussions, exchange knowledge, and collaboratively craft the future trajectory of regulated IHC assays.
26 October, Thursday, 4 pm CET/10 am EST
Precision medicine demands precision pathology. IHC from a Stain to an Assay – The TIME is NOW.

Clive Taylor
Emeritus Professor at Keck School of Medicine, USA
Read Clive's bio
Dr Clive Taylor is an Emeritus Professor of Pathology at the Keck School of Medicine, University of California. He is a pioneer in immunohistochemical (IHC) techniques for diagnosing surgical pathology, originally adapting these methods in Oxford, England, in 1972. He established a laboratory at the University of Southern California focused on lymphoma research and diagnosis. Dr Taylor has also served as a Trustee and President of the Biological Stain Commission (BSC), collaborating with the FDA to develop guidelines for IHC reagents and enhancing reproducibility.
Read the abstract
Immunohistochemistry (IHC) entered the realm of Diagnostic Surgical Pathology as more specific type special stain. Today IHC is applied in that role in Anatomic Pathology laboratories worldwide. With the advent of actionable molecular biomarkers, IHC was pressed into service for detection and then quantification of such markers where the IHC result alone could determine therapy. In the latter application IHC was ‘repurposed' from a stain to an assay. Unfortunately, the necessary pre-analytic, analytic and post-analytic changes did not evolve apace, resulting in poor clinical performance. Key technologies have now evolved to the point that deficits may now be addressed, thereby transmuting of IHC from a stain to an assay - "In Situ Proteomics."
16 November, Thursday, 4 pm CET/10 am EST
NordiQC: External Quality Assessment – Advancing Diagnostic Immunohistochemistry

Dr Rasmus Røge, MD, PhD
Hematopathologist and Clinical Assoc. Prof, Aalborg University
Read Rasmus' bio
Dr Rasmus Røge is a pathologist and Clinical Associate Professor affiliated with Aalborg University. His daily work focuses on hematopathology, specializing in the study and diagnosis of blood-related disorders. Dr Røge has been actively involved in the Nordic Immunohistochemical Quality Control (NordiQC) since 2012, an international external quality assessment scheme that serves diagnostic pathology laboratories worldwide. With more than 30 publications, his research primarily centers around quality assessment, methodology in Immunohistochemistry and introduction, validation and optimization of new biomarkers in pathology.
Read the abstract
Ensuring the accuracy and reliability of laboratory testing is paramount. This talk explores the vital role played by NordiQC (Nordic Immunohistochemical Quality Control) in enhancing the quality and standardization of diagnostic testing in pathology laboratories. I will discuss NordiQC's objectives, operational approach, and the outcomes it has yielded. Additionally, I will introduce several valuable tools for IHC laboratories, which can be accessed on the NordiQC website.
30 November, Thursday, 4 pm CET/10 am EST
Introducing QualitopixTM

Dirk Vossen
CDO, Visiopharm, the Netherlands
Read Dirk's bio
Dirk Vossen leads a cross-functional team to develop diagnostic and clinical applications of digital pathology. His track record of creating value through innovation in digital and computational pathology spans the entire range of development, from ideation through validation and certification of medical devices, as well as commercialization strategies.
Read the abstract
Dirk Vossen will introduce Visiopharm’s Qualitopix solution, which allows the monitoring and documentation of staining consistency. Learn about how easy Qualitopix fits into your workflow without the need for installation or integration. Be ready for new demands and regulations as the community is seeing the need for more regulation on IHC staining especially for predictive purposes. Dirk will also show the results of the first Qualitopix customers, who were able to identify unexpected shifts in their staining intensities. The labs already identified different reasons for the inconsistent staining, which will be explained.
14 December, Thursday, 4 pm CET/10 am EST
AI-based quantitative QA of IHC staining – The Henry Ford Health Experience

Omar Baba
MD, Clinical Pathologist, Henry Ford Hospital, USA
Read Omar's bio
Omar Z. Baba, M.D. is a Pathology Informatics Fellow at Henry Ford Health, Detroit, mentored by Dr J Mark Tuthill. A Clinical Pathologist trained at the American University of Beirut Medical Center in Lebanon, he has been at the forefront of pathology informatics, deeply engaged in multiple informatics operations at the department of Pathology and Lab medicine at HFH and notably leading a validation initiated on an AI-driven image analysis tool, which he presented at the 2023 PI Summit in Pittsburgh.
Read the abstract
Henry Ford Health had implemented Qualitopix to investigate their staining consistency of ER, PR, HER2, Ki-67 and PD-L1. Dr. Omar Baba will show the results of the analysis of 1340 slides and how the monitoring using Qualitopix supported the lab to uncover unexpected shifts in their staining consistency.
11 January, Thursday, 4 pm CET/10 am EST
Why Cell lines?

Colin Tristram
CEO, Histocyte, UK
Read Colin's bio
Colin has spent the last twenty years developing, marketing, and commercializing a range of products for the IVD market, specifically tissue diagnostics. Colin is the founder and current CEO of HistoCyte laboratories. He studied Medical Microbiology at Newcastle University, specializing in immunology. His MSc focused on HER2 in breast cancer, involving the development of procedures for IHC controls. With this knowledge he and his colleagues started HistoCyte and developed a range of high-quality, reproducible, and cost-effective analyte control material for same-slide use in histopathology. HistoCyte was acquired by Atlas Antibodies in 2021.
Read the abstract
In this webinar Colin will talk about the important role of cell lines together with Qualitopix in achieving staining consistency.
18 January, Thursday, 4 pm CET/10 am EST
How to achieve consistently accurate IHC test results

Steve Bogen
CEO, Boston Cell Standards, USA
Read Steve's bio
Dr Bogen is a Board-Certified Clinical Pathologist, having graduated from the University of Chicago Pritzker School of Medicine (M.D.) and the Weizman Institute of Science (Ph.D.), with a post-graduate residency and research fellowship at the Brigham and Women’s Hospital. He is Adjunct Professor of Pathology and Laboratory Medicine at Tufts University School of Medicine and served as Medical Director, Clinical Chemistry Laboratory at Tufts Medical Center until 2021. He is also an NCI-funded investigator whose research has focused principally on developing better diagnostic tools. Dr. Bogen was previously the founder of CytoLogix Corporation. He now serves as CEO of Boston Cell Standards.
Read the abstract
Published studies consistently demonstrate discrepancies in immunohistochemistry (IHC) staining from one laboratory to the next. In practice, clinical IHC laboratories usually report consistent results on samples that are negative or strongly positive. However, tumor samples with biomarker expression levels in the middle, between these two extremes, yield variable results among laboratories. If the IHC test is for a targeted therapeutic, then these are the patients at greatest risk to be treated incorrectly. The answer is to adopt the kind of quality assurance that is used in other parts of the clinical laboratory. In this seminar, we describe the theory and practice of new IHC quality assurance tools. Calibrators provide, for the first time, a direct readout on the analytic sensitivity of IHC assays. Achieving a target lower limit of detection (LOD) guarantees an appropriate stain intensity. Controls, on the other hand, are well-established in IHC. However, labs are usually not aware if their controls have relevant biomarker concentrations, including whether the biomarker concentration is even in the assay dynamic range. This presentation includes a description of both the science and clinical use of IHC calibrators and linear-range controls, including their objective quantification and use for statistical process control. These more advanced quality assurance tools are required to ensure accuracy and consistency in patient testing.
25 January, Thursday, 4 pm CET/10 am EST
Standardizing Ki-67 assays using cell lines

Regan Fulton
CEO, Array Science, USA
Read Regan's bio
Dr Fulton received his MD and PhD from the University of Minnesota and completed his residency in Anatomic Pathology at Stanford University. Following residency, he completed fellowships in Surgical Pathology and Immunodiagnosis at Stanford University and is board-certified in Anatomic Pathology. He is the founder and CEO of Array Science, LLC, a manufacturer of control and proficiency-testing material. He holds multiple patents for making tissue and cell culture microarrays. He now works full-time at Array Science, while providing pathology support in the development of diagnostics, as well as various phases of clinical trials. Dr Fulton has served as a consultant and paid speaker for several pharmaceutical and biotechnology companies.
Read the abstract
Ki-67 is a widely used marker of cell proliferation. In his talk, Regan Fulton will give an overview of the biological function of the Ki-67 protein and its utility in different clinical indications. He will demonstrate current problems with the assay’s reproducibility and suggest solutions to improve the standardization of the assay using cell lines.
8 February, Thursday, 4 pm CET/10 am EST
Inter-laboratory HER2 variability and daily quality control

Nils 't Hart
Pathologist, Isala Hospital, The Netherlands
Read Nils' bio
Nils ‘t Hart is a thoracic pathologist with a position at Isala since 2009. Isala is a top clinical hospital in the center of The Netherlands. Since Nils finished his residency at UMCG, he is focusing on diagnostics in thoracic pathology, molecular diagnostics and predictive immunohistochemistry. He is interested in new developments in oncology, how to implement predictive markers in daily practice using AI and eager to do pathology research. Last but not least, he is always looking for new innovative methods to teach about his passion.
Read the abstract
In this lecture, Dr. Hart will present a study on inter-laboratory HER2 variability and daily quality control across 40 Dutch pathology laboratories. He will explore the use of calibrators and cell lines, emphasize the importance of revalidation of HER2 tests, and discuss the advantages of Qualitopix as an AI-assisted tool for detecting staining inconsistencies in laboratories.
22 February, Thursday, 4 pm CET/10 am EST
Monitoring immunohistochemical stain quality with artificial intelligence

Paul J van Diest
Professor, UMC Utrecht, The Netherlands
Read Paul's bio
Paul J van Diest studied Medicine and did his PhD and pathology residency at VU University Medical Center in Amsterdam. After obtaining his Board certification (1996) he became Consultant Pathologist, Associate Professor (1999) and full Professor (2001). Since 2003 he is Head of the Department of Pathology at University Medical Center Utrecht. This department has gone fully digital and at the moment is involved in AI-research and implementation. He is Adjunct Professor of Oncology at the Sidney Kimmel Oncology Center at Johns Hopkins, Baltimore, USA, serves on the editorial board of international journals, and has been active in several international societies.
Read the abstract
UMC Utrecht has established an advanced digital pathology set up in their pathology lab. In this webinar, Professor van Diest will present the results of an extensive IHC stain and stainer evaluation performed using Qualitopix. The quantification of stain consistency enabled the lab to significantly improve their stain quality for HER2 and unveil staining variations based on their stainer.
7 March, Thursday, 4 pm CET/10 am EST
The Future of IHC: Opportunities and Challenges

Prof. Ralf Huss
BioM Biotech Cluster Dev. GmbH & University Hospital Augsburg, Germany
Read Ralf's bio
Ralf Huss is a Professor of Pathology and currently the Managing Director and CEO of the Biotechnology Development Agency in Munich, Germany. Prior to this role, he was the founding director of the Institute for Digital Medicine at the University Hospital Augsburg, Germany. Dr. Huss is board-certified in anatomical, experimental, and molecular pathology, with over 30 years of experience in international academic institutions and the pharmaceutical industry with a focus on histopathology, immunology, cancer research, and digital medicine.
Read the abstract
In his lecture, Dr. Huss will summarize the previous highlights of this extraordinary Masterclass from the need for quality control in precision pathology to the implementation of an automated and AI-based solution in a routine pathology workflow. With a special emphasis on the value of AI in controlling the robust performance of standardized IHC assays, Dr. Huss will advocate the importance to IHC tests to be acknowledged as an assay without increasing the burden on routine histopathology laboratories. On the contrary, such an approach could even compensate or at least forecast assay failure which allows early countermeasures from pre-analytical to post-analytical errors.

Clive Taylor
Emeritus Professor at Keck School of Medicine, USA

Dr Rasmus Røge, MD, PhD
Hematopathologist and Clinical Associate Professor, Aalborg University
Dr Rasmus Røge is a pathologist and Clinical Associate Professor affiliated with Aalborg University. His daily work focuses on hematopathology, specializing in the study and diagnosis of blood-related disorders. Dr Røge has been actively involved in the Nordic Immunohistochemical Quality Control (NordiQC) since 2012, an international external quality assessment scheme that serves diagnostic pathology laboratories worldwide. With more than 30 publications, his research primarily centers around quality assessment, methodology in Immunohistochemistry and introduction, validation and optimization of new biomarkers in pathology.

Prof Ralf Huss
BioM Biotech Cluster Development GmbH & University Hospital Augsburg, Germany
Ralf Huss is a Professor of Pathology and currently the Managing Director and CEO of the Biotechnology Development Agency in Munich, Germany. Prior to this role, he was the founding director of the Institute for Digital Medicine at the University Hospital Augsburg, Germany. Dr. Huss is board-certified in anatomical, experimental, and molecular pathology, with over 30 years of experience in international academic institutions and the pharmaceutical industry with a focus on histopathology, immunology, cancer research, and digital medicine.

Omar Baba
MD, Clinical Pathologist, Henry Ford Hospital, USA
Omar Z. Baba, M.D. is a Pathology Informatics Fellow at Henry Ford Health, Detroit, mentored by Dr J Mark Tuthill. A Clinical Pathologist trained at the American University of Beirut Medical Center in Lebanon, he has been at the forefront of pathology informatics, deeply engaged in multiple informatics operations at the department of Pathology and Lab medicine at HFH and notably leading a validation initiated on an AI-driven image analysis tool, which he presented at the 2023 PI Summit in Pittsburgh.

Nils 't Hart
Pathologist, Isala Hospital, The Netherlands

Paul J van Diest
Professor, UMC Utrecht, The Netherlands

Colin Tristram
CEO, Histocyte, UK

Steve Bogen
CEO, Boston Cell Standards, USA

Regan Fulton
CEO, Array Science, USA
Dr Fulton received his MD and PhD from the University of Minnesota and completed his residency in Anatomic Pathology at Stanford University. Following residency, he completed fellowships in Surgical Pathology and Immunodiagnosis at Stanford University and is board-certified in Anatomic Pathology. He is the founder and CEO of Array Science, LLC, a manufacturer of control and proficiency-testing material. He holds multiple patents for making tissue and cell culture microarrays. He now works full-time at Array Science, while providing pathology support in the development of diagnostics, as well as various phases of clinical trials. Dr Fulton has served as a consultant and paid speaker for several pharmaceutical and biotechnology companies.

Michael Grunkin
CEO, Visiopharm, Denmark

Dirk Vossen
CDO, Visiopharm, the Netherlands
Dirk Vossen leads a cross-functional team to develop diagnostic and clinical applications of digital pathology. His track record of creating value through innovation in digital and computational pathology spans the entire range of development, from ideation through validation and certification of medical devices, as well as commercialization strategies.
Posters being presented at AACR
Session Date and Time: Sunday Apr 16, 2023 1:30 PM - 5:00 PM
Published Abstract Number: 619
Poster Board Number: 20
Poster Section: 21
Authors: Y. Shi et al. from Xi'an Jiaotong University, China,; with MD Anderson Cancer Center
Session Date and Time: Sunday Apr 16, 2023 1:30 PM - 5:00 PM
Published Abstract Number: 1018
Poster Board Number: 28
Poster Section: 41
Authors: D. Zielinski et al. from Discovery Life Sciences
Session Date and Time: Monday Apr 17, 2023 1:30 PM - 5:00 PM
Published Abstract Number: 2322
Poster Board Number: 3
Poster Section: 45
Authors: B. Dennison et al. from Lanterne Dx
Session Date and Time: Tuesday Apr 18, 2023 1:30 PM - 5:00 PM
Published Abstract Number: 3588
Poster Board Number: 13
Poster Section: 3
Authors: M. Pore et al. from NCI Frederick, MD
Session Date and Time: Tuesday Apr 18, 2023 1:30 PM - 5:00 PM
Published Abstract Number: 4625
Poster Board Number: 15
Poster Section: 4
Authors: B. O’Neill et al. from Visiopharm; with Standard Bio Tools