Standardize your tissue biomarker staining effortlessly with a complete workflow from scanning to documentation
25-30%
of laboratories fail to meet minimum proficiency standards for critical predictive markers2.
2-3 times
EQA runs happen typically 2-3 times a year, making consistent IHC staining maintenance difficult
10x
higher error rates. For decades, the reported error rates of IHC staining have been 10 times higher than error rates for other clinical tests, risking or causing wrong diagnosis.
Trust your staining through AI driven quantification and monitoring
Request a demo today

A complete, intuitive workflow from scanning to documentation
With the integrated Qualitopix® solution of software and slide scanner, you gain a seamless and accessible pathway to staining standardization.

Get started easily with a frictionless setup
With no need for extensive installation nor digital infrastructure, you will quickly be up and running. Simply connect, start scanning slides and be automatically notified if they are outside of range.
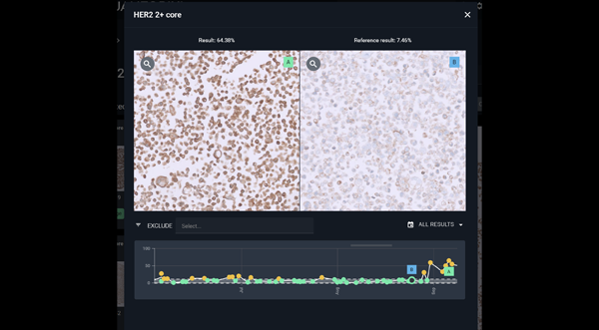
Trust your staining through AI-driven quantification and monitoring
Qualitopix enables continuous measuring, monitoring, and documentation of staining consistency, promptly identifying, and addressing any deviations.

High quality images, easily
The Qualitopix solution includes a cutting-edge slide scanner powered by Grundium that sets new standards for compact digital microscopy through an unparalleled combination of exceptional image quality, user-friendly operation, and cost-effectiveness.
"The present figures concerning IHC testing accuracy are worrying. The direct link between test precision and patient outcomes cannot be overstated. This joint initiative, combining Visiopharm's AI-driven internal quality control with our external quality assessment, aims to significantly enhance testing quality, a critical factor in delivering optimal patient care."
- Mr. Andy Dodson, Director of UK NEQAS ICC & ISH
Request a demo today
This is how Qualitopix works
With no need for extensive installation nor digital infrastructure, you will quickly be up and running. Simply connect to your PC, start scanning slides and be automatically notified if they are outside of range.
#1
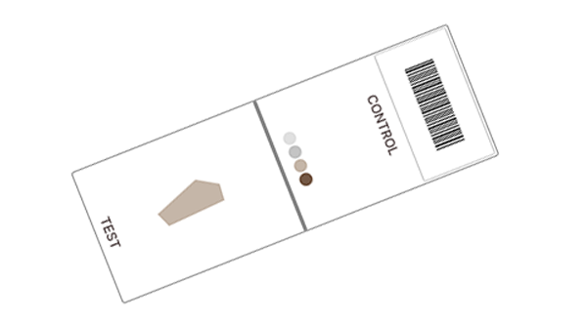
Purchase standardized
reference material
Cell line (Histocyte, ArrayScience)
Coming soon: Microbead controls and calibrators (BCS).
#2
Place on slides and stain
Follow your routine staining protocol.
#3

Scan slides
Slides are scanned with the Qualitopix slide scanner
#4
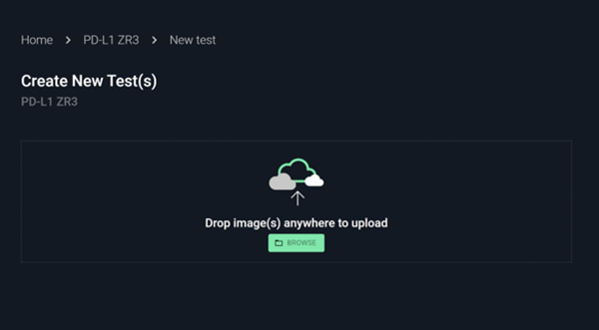
Upload images and metadata
No installation required. Just drag and drop the images with key metadata.
#5

Receive Quantitative results
Shortly after upload, you will receive results and will be notified if a test requires your attention.
#6

Troubleshoot
If you do encounter outliers, Qualitopix provides tools to streamline the troubleshooting process and identify the root cause.
Resources
Here are some publications/papers about Qualitopix
Article
Qualitopix: Artificial intelligence-based quantitative quality assurance of immunohistochemistry staining-The Henry Ford Health experience
Publication
Improving PD-L1 quality control using a dynamic range cell line and Qualitopix analysis
Publication
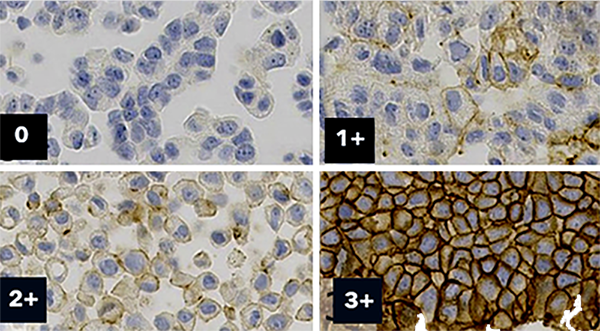
Inter-laboratory variability of HER2 low analysis using artificial intelligence
“Our analysis revealed a 26-34% rate of outliers at 1 SD for the five stains, 10 times higher than the currently reported number from our lab.” Omar Baba, American Journal of Clinical Pathology - Qualitopix: Artificial intelligence-based quantitative quality assurance of immunohistochemistry staining-The Henry Ford Health experience
References
1 Vyberg M et al. Immunohistochemical expression of HER2 in breast cancer: socioeconomic impact of inaccurate tests. BMC Health Serv Res., 15:352, August 2015 Read full paper
2 Nielsen S et al., Lessons Learned, Challenges Taken, and Actions Made for “Precision” Immunohistochemistry. Analysis and Perspectives From the NordiQC Proficiency Testing Program. Applied Immunohistochemistry & Molecular Morphology, 31(7):p 452-458, August 2023 Read full paper

Clive Taylor
Emeritus Professor at Keck School of Medicine, USA

Dr Rasmus Røge, MD, PhD
Hematopathologist and Clinical Associate Professor, Aalborg University
Dr Rasmus Røge is a pathologist and Clinical Associate Professor affiliated with Aalborg University. His daily work focuses on hematopathology, specializing in the study and diagnosis of blood-related disorders. Dr Røge has been actively involved in the Nordic Immunohistochemical Quality Control (NordiQC) since 2012, an international external quality assessment scheme that serves diagnostic pathology laboratories worldwide. With more than 30 publications, his research primarily centers around quality assessment, methodology in Immunohistochemistry and introduction, validation and optimization of new biomarkers in pathology.

Prof Ralf Huss
BioM Biotech Cluster Development GmbH & University Hospital Augsburg, Germany
Ralf Huss is a Professor of Pathology and currently the Managing Director and CEO of the Biotechnology Development Agency in Munich, Germany. Prior to this role, he was the founding director of the Institute for Digital Medicine at the University Hospital Augsburg, Germany. Dr. Huss is board-certified in anatomical, experimental, and molecular pathology, with over 30 years of experience in international academic institutions and the pharmaceutical industry with a focus on histopathology, immunology, cancer research, and digital medicine.

Omar Baba
MD, Clinical Pathologist, Henry Ford Hospital, USA
Omar Z. Baba, M.D. is a Pathology Informatics Fellow at Henry Ford Health, Detroit, mentored by Dr J Mark Tuthill. A Clinical Pathologist trained at the American University of Beirut Medical Center in Lebanon, he has been at the forefront of pathology informatics, deeply engaged in multiple informatics operations at the department of Pathology and Lab medicine at HFH and notably leading a validation initiated on an AI-driven image analysis tool, which he presented at the 2023 PI Summit in Pittsburgh.

Nils 't Hart
Pathologist, Isala Hospital, The Netherlands

Paul J van Diest
Professor, UMC Utrecht, The Netherlands

Colin Tristram
CEO, Histocyte, UK

Steve Bogen
CEO, Boston Cell Standards, USA

Regan Fulton
CEO, Array Science, USA
Dr Fulton received his MD and PhD from the University of Minnesota and completed his residency in Anatomic Pathology at Stanford University. Following residency, he completed fellowships in Surgical Pathology and Immunodiagnosis at Stanford University and is board-certified in Anatomic Pathology. He is the founder and CEO of Array Science, LLC, a manufacturer of control and proficiency-testing material. He holds multiple patents for making tissue and cell culture microarrays. He now works full-time at Array Science, while providing pathology support in the development of diagnostics, as well as various phases of clinical trials. Dr Fulton has served as a consultant and paid speaker for several pharmaceutical and biotechnology companies.

Michael Grunkin
CEO, Visiopharm, Denmark

Dirk Vossen
CDO, Visiopharm, the Netherlands
Dirk Vossen leads a cross-functional team to develop diagnostic and clinical applications of digital pathology. His track record of creating value through innovation in digital and computational pathology spans the entire range of development, from ideation through validation and certification of medical devices, as well as commercialization strategies.
Posters being presented at AACR
Session Date and Time: Sunday Apr 16, 2023 1:30 PM - 5:00 PM
Published Abstract Number: 619
Poster Board Number: 20
Poster Section: 21
Authors: Y. Shi et al. from Xi'an Jiaotong University, China,; with MD Anderson Cancer Center
Session Date and Time: Sunday Apr 16, 2023 1:30 PM - 5:00 PM
Published Abstract Number: 1018
Poster Board Number: 28
Poster Section: 41
Authors: D. Zielinski et al. from Discovery Life Sciences
Session Date and Time: Monday Apr 17, 2023 1:30 PM - 5:00 PM
Published Abstract Number: 2322
Poster Board Number: 3
Poster Section: 45
Authors: B. Dennison et al. from Lanterne Dx
Session Date and Time: Tuesday Apr 18, 2023 1:30 PM - 5:00 PM
Published Abstract Number: 3588
Poster Board Number: 13
Poster Section: 3
Authors: M. Pore et al. from NCI Frederick, MD
Session Date and Time: Tuesday Apr 18, 2023 1:30 PM - 5:00 PM
Published Abstract Number: 4625
Poster Board Number: 15
Poster Section: 4
Authors: B. O’Neill et al. from Visiopharm; with Standard Bio Tools
